
Aluminum Foil: Burn or Melt?

Purpose: In the world of chemistry, certain elements will behave differently when exposed to certain circumstances. This experiment will peer into the element Aluminum to see whether it will burn or melt when exposed to a flame.
![]()
Hypothesis: The hypothesis of this experiment is that Aluminum will melt when exposed to flames. This judgement is based on the usual behavior of metals that are exposed to flame. Metals on occasion do burn, one example is magnesium, however, the hypothesis stands on the fact that aluminum isn't one of these freak metals.
![]()
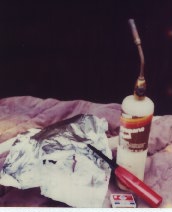
Equipment: For this experiment, the necessary equipment include aluminum foil, matches or a butane lighter, a blow torch and pen and paper.
![]()
Procedure: The aluminum foil will be put through two different heats of flames. The first is by a match or butane lighter. The first step is to cut the aluminum foil into two large squares. With one of the squares, light the edge of the aluminum, observe results. Next step is to hold the aluminum foil so that the flame can be placed underneath it, again, observe results. Repeat the process with the blow torch. **************caution is advised****************
![]()
Observations:

Butane Lighter
| Process Done to Aluminum Foil | Observations |
| Corner of Aluminum foil was exposed to flame | When the aluminum foil was exposed to the flame, the aluminum foil would shrink away towards the middle. The edge of the aluminum foil would appear red as it touched the flame. As flame was removed, the aluminum foil rapidly decreased temperature and become brittle. |
| Middle of aluminum foil square was heated | The aluminum foil became extremely oxidized and would crinkle and fold up a little. However, the aluminum foil was relatively unchanged by the heat of the butane lighter. |

Blow Torch
| Process Done to Aluminum Foil | Observations |
| Corner of Aluminum foil was exposed to flame | When aluminum foil was exposed to the blow torch, the foil would move rapidly away from the edge toward the center. The aluminum foil would also become crinkled up as this happened. Foil was red hot during exposure to the flame, however, afterwards the foil was excessively brittle. |
| Middle of aluminum foil square was heated | As flame was exposed to the center of the foil, a round circle of red hot aluminum foil formed, when the flame was removed, the aluminum foil was very brittle in the center, and it was translucent. |
![]()
Conclusion: The question on whether or not the foil burned is a question of whether it was physically or chemically changed. The observations indicate that the aluminum foil burned as opposed to melted. The fact that the aluminum foil became brittle indicates that the aluminum foil lost it's properties as aluminum foil, and therefore had to be a completely different substance. Unfortunately for the hypothesis of this experiment, the results indicate that the aluminum foil had been chemically changed and therefore had been burned.
Note: The above song playing is Coiled's Depression.