
![]()
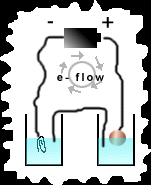
Hypothesis: It is the hypothesis of this experiment that if the proper items are collected with which to construct a Voltaic cell (pictured at top and in procedure), then it should be possible to copper plate objects using items found around the house.
![]()
Equipment: Needed for this experiment is a 1.5v battery (AA size), Aluminum foil, Dixie cups (small size), cloth string, a penny, salt, water, and Copper (II) Sulfate. Copper (II) Sulfate is sometimes used to purify the water in fish tanks although it is known to be toxic to humans in several ways. Caution is advised while using this item. It should be able to be found where fish tank supplies are sold.
![]()
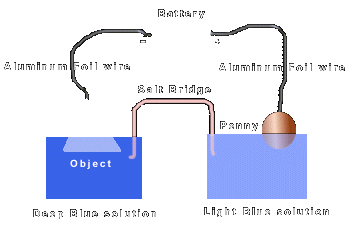
Procedure: The first step is to roll the aluminum foil into 2 separate 'wires'. With one wire, attach one end to the positive end of the battery and the other end to a copper penny. The other wire attaches to the negative end of the battery and its other end attaches to the object to be copper plated. The next step is to fill the two dixie cups half full of water. Place a good amount of Copper (II) Sulfide into one of the cups so that it takes a deep blue color after stirring. Into the other cup place very little so that it is light blue in color. Place the object to be copper plated into the deep blue solution and the penny into the light blue solution. The final step is to create a salt bridge which will 'complete the circuit'. This is done by taking a string and rolling it in salt and then creating a solution of salt water. Place the string so that one end comes in contact with the deep blue solution and the other end with the light blue solution. slowly drip salt water on the 'salt bridge' while the experiment progresses. After one to two hours, check the object to see if copper plating has occurred. See below diagram to see the above illustrated.
![]()
Results: After 2.5 hours copper was found on the object (paper clip).
![]()
Conclusion: The results indicate that copper plating was possible utilizing household equipment. Ultimately, further research will be required to determine whether this can be done on non electric conducting substances. However, this experiment does demonstrate that the secrets of metal plating objects can be obtained by anyone.